Background: Most individuals diagnosed with indolent B-cell non-Hodgkin lymphoma (iB-NHL) should be expected to have a normal or near normal life span (Maurer et al., 2016). Thus, a major goal is identifying treatments that maintain efficacy while reducing toxicity and improve ease of administration. Window-of-opportunity studies are well-suited to evaluate the likely ceiling of activity of single agent therapies - assuming highest efficacy may be observed prior to emergence of resistance mechanisms or cumulative host toxicities. We hypothesized that oral ixazomib (Ix) would be safe and effective in untreated iB-NHL based on safety data in myeloma and efficacy data with the intravenous formulation (Assouline et al., 2014), and sought to evaluate it in the frontline setting both as a single agent (with a lead-in period) and together with rituximab (R).
Methods: This single-arm, open-label phase II investigator-initiated trial (NCT 02339922) was conducted at the University of Washington / Fred Hutch Cancer Research Center / Seattle Cancer Care Alliance. Eligibility included an indication for treatment of iB-NHL per National Comprehensive Cancer Network guidelines, ECOG ≤ 2, and radiographically measurable disease. Prior standard systemic treatment of iB-NHL was permitted only for cases of mucosa-associated marginal zone lymphoma (MZL) relapsed after or refractory to antibiotics. Ix was administered at 4 mg orally once a week on consecutive 28-day cycles. A single course of 4 weekly doses of R at 375 mg/m2 was added during the 7th cycle, closing the window period; Ix alone was continued until disease progression or unacceptable toxicity. The primary endpoint was investigator-assessed overall response rate (ORR) after independent radiology review. Response assessment occurred at every 2 cycles using standard (Lugano) criteria.
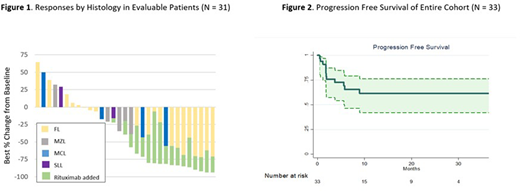
Results: Between February 2017 and January 2020 a total of 33 patients began treatment. The median age was 62 years (range 38 to 85) and 67% were men. Histologic subtypes included follicular lymphoma (FL, n = 20), MZL (n = 7), mantle cell lymphoma (MCL, n = 4), and small lymphocytic lymphoma (SLL, n = 2). The most common indications for therapy were bulky disease (42%) and symptoms due to lymphoma (27%). In cases of FL, 35% had > 6 cm tumor bulk and 20% had Follicular Lymphoma International Prognostic Index score ≥ 3. Median follow-up was 16.8 months (range 1.2 - 39.8). In the 6-month Ix-only window, the ORR was 24% for the entire cohort and 35% for FL [complete response (CR) rate 3% and 5%, respectively] (Figure 1). Reduction of disease was seen in 23 (70%), including 15 (75%) of FL, 4 (57%) of MZL, and 3 (75%) of MCL. Overall, the ORR was 45% and 60% for FL (CR rate 27% and 35%, respectively) as of June 1, 2020 (at which point 3 patients had yet to undergo evaluation post R). Progression free survival (PFS) at 2 years for all subjects was 62% and for those with FL was 69%; median PFS was not reached (Figure 2). For the 15 patients with objective response, the median time to response was 5.5 months (range 1.8 - 11.0) and the median duration of response was not reached (87% in remission at 2 yrs).
Adverse events (AEs) > grade 3 deemed related to treatment were not observed and such grade 3 events occurred in 5 unique patients (15%). Serious AEs were recorded in 2 patients (6%). Most AEs were grade 1-2 and included nausea (58%, typically only for few a few hours after the weekly dose), diarrhea (39%), headache (30%), and vomiting (30%). Peripheral neuropathy (PN) was reported by 12% (motor PN in 9% and sensory PN in 3%); all cases were grade 1 except one case (3%) of grade 2 motor PN. Toxicity from Ix resulted in dose-holds in 21%, dose-reduction to 3 mg weekly in 9%, and discontinuation in 6% (one case of grade 3 hyponatremia and one case of grade 2 confusion).
Conclusion: Once weekly oral Ix has a favorable safety profile and shows considerable activity in frontline treatment of iB-NHL, with the best results in FL. Combined with a single 4-week course of R, Ix can achieve durable disease control with very low toxicity in a majority of patients with FL, representing a convenient regimen amenable to remote management if indicated. This approach has the potential to support the overall strategy of lowering the burden of treatment while maintaining expected excellent outcomes in most patients with FL.
Graf:TG Therapeutics: Research Funding; BeiGene: Research Funding; MorphoSys: Consultancy; Acerta Pharma: Research Funding. Lynch:TG Therapeutics: Research Funding; Genentech: Research Funding; Juno Therpeutics: Research Funding; Incyte: Research Funding; Bayer: Research Funding; MorphoSys: Consultancy; Cyteir: Research Funding; Rhizen Pharmaceuticals: Research Funding; Takeda: Research Funding. Ujjani:MorphoSys: Consultancy; Genentech: Consultancy, Honoraria; Atara: Consultancy, Honoraria; Gilead/Kite: Consultancy, Research Funding; Verastem Oncology: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding. Cowan:Bristol Myers Squibb: Research Funding; Sanofi: Consultancy; Cellectar: Consultancy; Abbvie: Research Funding; Janssen: Consultancy, Research Funding. Smith:Bristol Meyers Squibb: Research Funding; Incyte: Research Funding; Ayala: Research Funding; Bayer: Research Funding; AstraZeneca: Research Funding; Acerta Pharma BV: Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding; Portola: Research Funding; Seattle Genetics: Research Funding; AstraZeneca: Consultancy; Millenium/Takeda: Consultancy; Beigene: Consultancy; Karyopharm: Consultancy; De Novo Biopharma: Research Funding; Genentech: Research Funding; Ignyta: Research Funding. Shadman:Abbvie, Genentech, Astra Zeneca, Sound Biologics , Pharmacyclics, Verastem, ADC therapeutics, Beigene, Cellectar, BMS, Morphosys and Atara Biotherapeutics: Consultancy; Mustang Bio, Celgene, Pharmacyclics, Gilead, Genentech, Abbvie, TG therapeutics, Beigene, Astra Zeneca, Sunesis, Beigene: Research Funding. Godwin:Pfizer Inc.: Research Funding; Immunogen Inc.: Research Funding. Cassaday:Amgen: Consultancy, Research Funding; Kite/Gilead: Consultancy, Research Funding; Merck: Research Funding; Pfizer: Honoraria, Research Funding; Seattle Genetics: Current Employment, Current equity holder in publicly-traded company; Vanda Pharmaceuticals: Research Funding. Fromm:Merck: Research Funding. Gopal:Seattle Genetics; Janssen; IMab Bio; TG Therapeutics; Astra Zeneca; Merck; Gilead; ADC Therapeutics; Nurix; TG therapeutics, Cellectar; Actinium: Consultancy; Seattle Genetics; Janssen; Takeda; IgM Bio; IMab Bio; BMS; Astra Zeneca; Merck; Gilead: Research Funding; imab bio, takeda,astrazeneca,gilead: Research Funding; IgM bio, BMS, merck: Research Funding.
Ixazomib has not been approved for use in treating indolent B-NHL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal